Difference between revisions of "Polyelectrolyte Multilayers"
| (58 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
| − | + | == What is a Polyelectolyte Multilayer (PEM)? == | |
| − | '' | + | <onlyinclude>PEMs are composed of '''alternating layers of oppositely charged polyelectrolytes''' (PEs) (synthetic PEs or biomolecules), which are generally built up based on the Layer-by-Layer technique. [1,2] Due to their potential applications as membrane, encapsulation and matrix materials, and for enzymes and proteins in sensor applications, PEMs have stimulated great interests from both academic researchers and industries.[3] See also a [http://www.chem.fsu.edu/multilayers/ PEM website]. Despite the large number of experimental works, '''theoretical and computational studies''' toward understanding the microscopic structure of PEMs '''are scarce'''.[4] |
| + | </onlyinclude> | ||
| + | == Our Research == | ||
| − | + | In close collaboration with experimental investigations from groups of Prof. [http://www.chemie.tu-berlin.de/klitzing/menue/home/ von Klitzing] and Prof. [http://www.bio.ph.tum.de/index.php?id=69 Hugel], we are currently working to investigate the inner structure and dynamics of a small layer number of PEMs via '''all-atom''' (AA) and '''coarse-grained''' (CG) simulations. The project is funded by the [http://www.dfg-spp1369.de/front/joomla/ '''DFG SPP 1369''': Polymer-Solid Contacts: Interfaces and Interphases] | |
| + | [[Image:spp1369_logo_small.jpg|100px|]] | ||
| − | |||
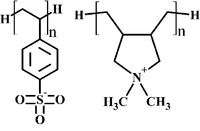
| − | + | [[Image:scheme_PSS-PDADMA.png|200px|right|thumb|Schematic representation of PSS and PDADMA.]] | |
| − | + | The AA level simulations proved to be '''consistent''' with existing experimental data on '''chain conformation''' of adsorbed poly(styrene sulfonate) (PSS) in PSS monolayer systems, '''dielectric permittivity''' and '''diffusion constant''' of water in PSS/PDADMA polyelectrolyte complexes (PDADMA stand for poly(diallyldimethylammonium)). | |
| + | So far, we are building the PSS/PDADMA bilayers based on the previously obtained PSS monolayers and we are expecting to extract exciting information from these studies. The simulation of a bilayer represents our final goal for atomistic simulations of PMEs so far, due to the high requirement of computer resources (ca. 1000 000 cpu hours in total). | ||
| − | + | Due to the limitations of atomistic simulations, further insight into structure and dynamics of PEMs can be achieved only with simulations at CG level. Qualitative understanding and agreement with experiments has been obtained by us using the already existing generic bead-spring PE model. However, a refined CG PE model is needed in order to be quantitatively predictive. This is part of our next working program. | |
| − | + | Some selected results obtained during the last 2.5 years are: | |
| − | + | '''a) Bilayer thermodynamical instability''' | |
| − | [ | + | [[Image:thermodynamic_stability_bilayer_vs_trilayer.png|200px|right|thumb|Thermodynamic instability of the bilayer and the stability of the fast deposited tri-layer.]] |
| − | + | Our CG level simulations have shown that depending on the relative strength of the monomer-monomer and monomer-surface interaction energies, a '''progressive redissolution''' of the first bilayer or a '''partial dewetting''' resulting in a disordered melt can happen. | |
| − | + | We have shown that a '''fast enough deposition of the third layer''' -- before the aging process -- can '''prevent such redissolution or partial dewetting''' and provide the stability | |
| + | needed to form a PEM. We have checked that the deposition of further layers is a stable process. This suggests that the first PE bilayer is not thermodynamically stable, while tri-layers and higher layers are stable, at least within the long run time of our simulations. | ||
| − | + | '''b) Charge compensation mechanism''' | |
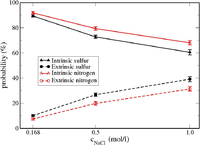
| − | [ | + | [[Image:intrinsic_vs_extrinsic_result_in_PEC.png|200px|right|thumb|Possibilities of sulfurs from PSS and nitrogens from PDADMA which are intrinsically and extrinsically charge compensated.]] |
| − | + | In our AA level simulations on PSS/PDADMA complexes, intrinsic (polyanions pair with polycations) and extrinsic (polyions pair with salt ions) charge compensation mechanisms have been found to co-exist, although '''the intrinsic one is predominant''' in the investigated salt (NaCl) concentration range from 0.17 to 1.00 mol/L. | |
| − | + | Furthermore, the relative scale of the interaction energy of the ion-pairs in such PSS/PDADMA mixture is calculated to follow (in kJ/mol): Na-Cl (-520) > PSS-Na (-420) > PDADMA-Cl (-280) ~ PSS-PDADMA (-270). The relative scale of the interaction energy can be very useful to explain some experimental finding, such as PSS is found to be in a higher concentration than PDADMA in PSS/ PDADMA complexes [5]. This information is also valuable to properly model the interactions between ion-pairs in the upcoming refined CG model. | |
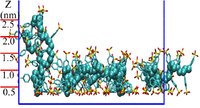
| − | [ | + | '''c) PSS adsorption monolayer''' |
| + | [[Image:PSS_momolayer.png|200px|right|thumb|PSS adsorption monolayer]] | ||
| − | + | The PSS monolayer is diposited from a PSS solution via atomistic simulations. Our results demonstrate that short-range interactions of van der Waals origin from the adsorbing substrate play a significant role in the layer structure of the adsorbed PSS, and they alone are already sufficient to induce a stable PSS adsorption layer. The PSS chains are found to behave as hydrophilic PEs, two kinds of conformations of which are observed: flat PSS adsorption layer dominates with some adsorbed PSS chains dangling into the above PSS solution. | |
| − | |||
| − | + | '''d) PE chain pulling experiment''' | |
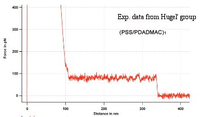
| − | [ | + | [[Image:PE_pulling_experiment.png|200px|thumb|Results from our CG simulations]] |
| + | [[Image:PE_pulling_experiment_Hugel.png|200px|thumb|Exp. data from Hugel's group]] | ||
| − | + | The present, non-refined CG model yields a qualitative agreement with the experiments by the Hugel group. This makes us confident that maybe even a quantitative comparison might be obtainable once the refined coarse-grained model will be ready. | |
| − | + | In the computer simulations on PE pulling experiments, a PE chain, which is similar to the PE chains of the capping layer, is introduced with the corresponding counterions. The averaged force, that is needed to keep one of the chain ends fixed at a given point Z_{tip}, is measured by performing several independent runs. The position of the chain tip is slowly increased to a new value where a new measurement was performed. | |
| − | + | == Scientists == | |
| − | [ | + | * ''[[Christian Holm]]'' |
| + | * ''[[Baofu Qiao]]'' | ||
| + | * ''[[Joan Josep Cerdà]]'' | ||
| + | * ''[[Marcello Sega]]'' | ||
| − | + | == Publications == | |
| − | + | <bibentry> messina03a, messina04b, cerda09b,cerda09c, qiao10a, qiao11a</bibentry> | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | [ | + | == References == |
| + | [1] J. Schmitt, G. Decher and G. Hong. ''Thin Solid Films'', '''1992''', ''210/211'', 831.[http://www.sciencedirect.com/science/article/B6TW0-47X13HN-47/2/e56caa8317193804cc1863ce3db4a32b URL] | ||
| + | [2] G. Decher, ''Science'','''1997''', ''277'', 1232. [http://www.sciencemag.org/cgi/content/abstract/277/5330/1232 URL] | ||
| − | + | [3] J. B. Schlenoff, ''Langmuir'', '''2009''', ''25'', 14007.[http://pubs.acs.org/doi/abs/10.1021/ja802054k URL] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | [4] A. V. Dobrynin. ''Curr. Opin. Colloid Interface Sci'', '''2008''', ''13'', 376.[http://www.sciencedirect.com/science/article/B6VRY-4S7JFXX-2/2/fd13c66c913bca456ed80d11acd1da39 URL] | ||
| − | + | [5] H. H. Hariri, and J. B. Schlenoff, ''Macromolecules'', '''2010''', DOI: 10.1021/ma1012978. [http://dx.doi.org/10.1021/ma1012978 URL] | |
| − | + | [[Category:Research]] | |
Latest revision as of 20:21, 5 September 2011
What is a Polyelectolyte Multilayer (PEM)?
PEMs are composed of alternating layers of oppositely charged polyelectrolytes (PEs) (synthetic PEs or biomolecules), which are generally built up based on the Layer-by-Layer technique. [1,2] Due to their potential applications as membrane, encapsulation and matrix materials, and for enzymes and proteins in sensor applications, PEMs have stimulated great interests from both academic researchers and industries.[3] See also a PEM website. Despite the large number of experimental works, theoretical and computational studies toward understanding the microscopic structure of PEMs are scarce.[4]
Our Research
In close collaboration with experimental investigations from groups of Prof. von Klitzing and Prof. Hugel, we are currently working to investigate the inner structure and dynamics of a small layer number of PEMs via all-atom (AA) and coarse-grained (CG) simulations. The project is funded by the DFG SPP 1369: Polymer-Solid Contacts: Interfaces and Interphases
![]()
The AA level simulations proved to be consistent with existing experimental data on chain conformation of adsorbed poly(styrene sulfonate) (PSS) in PSS monolayer systems, dielectric permittivity and diffusion constant of water in PSS/PDADMA polyelectrolyte complexes (PDADMA stand for poly(diallyldimethylammonium)).
So far, we are building the PSS/PDADMA bilayers based on the previously obtained PSS monolayers and we are expecting to extract exciting information from these studies. The simulation of a bilayer represents our final goal for atomistic simulations of PMEs so far, due to the high requirement of computer resources (ca. 1000 000 cpu hours in total).
Due to the limitations of atomistic simulations, further insight into structure and dynamics of PEMs can be achieved only with simulations at CG level. Qualitative understanding and agreement with experiments has been obtained by us using the already existing generic bead-spring PE model. However, a refined CG PE model is needed in order to be quantitatively predictive. This is part of our next working program.
Some selected results obtained during the last 2.5 years are:
a) Bilayer thermodynamical instability
Our CG level simulations have shown that depending on the relative strength of the monomer-monomer and monomer-surface interaction energies, a progressive redissolution of the first bilayer or a partial dewetting resulting in a disordered melt can happen.
We have shown that a fast enough deposition of the third layer -- before the aging process -- can prevent such redissolution or partial dewetting and provide the stability needed to form a PEM. We have checked that the deposition of further layers is a stable process. This suggests that the first PE bilayer is not thermodynamically stable, while tri-layers and higher layers are stable, at least within the long run time of our simulations.
b) Charge compensation mechanism
In our AA level simulations on PSS/PDADMA complexes, intrinsic (polyanions pair with polycations) and extrinsic (polyions pair with salt ions) charge compensation mechanisms have been found to co-exist, although the intrinsic one is predominant in the investigated salt (NaCl) concentration range from 0.17 to 1.00 mol/L.
Furthermore, the relative scale of the interaction energy of the ion-pairs in such PSS/PDADMA mixture is calculated to follow (in kJ/mol): Na-Cl (-520) > PSS-Na (-420) > PDADMA-Cl (-280) ~ PSS-PDADMA (-270). The relative scale of the interaction energy can be very useful to explain some experimental finding, such as PSS is found to be in a higher concentration than PDADMA in PSS/ PDADMA complexes [5]. This information is also valuable to properly model the interactions between ion-pairs in the upcoming refined CG model.
c) PSS adsorption monolayer
The PSS monolayer is diposited from a PSS solution via atomistic simulations. Our results demonstrate that short-range interactions of van der Waals origin from the adsorbing substrate play a significant role in the layer structure of the adsorbed PSS, and they alone are already sufficient to induce a stable PSS adsorption layer. The PSS chains are found to behave as hydrophilic PEs, two kinds of conformations of which are observed: flat PSS adsorption layer dominates with some adsorbed PSS chains dangling into the above PSS solution.
d) PE chain pulling experiment
The present, non-refined CG model yields a qualitative agreement with the experiments by the Hugel group. This makes us confident that maybe even a quantitative comparison might be obtainable once the refined coarse-grained model will be ready.
In the computer simulations on PE pulling experiments, a PE chain, which is similar to the PE chains of the capping layer, is introduced with the corresponding counterions. The averaged force, that is needed to keep one of the chain ends fixed at a given point Z_{tip}, is measured by performing several independent runs. The position of the chain tip is slowly increased to a new value where a new measurement was performed.
Scientists
Publications
-
René Messina, Christian Holm, Kurt Kremer.
Polyelectrolyte Multilayering in Spherical Geometry.
Langmuir 19:4473–4482, 2003.
[PDF] (963 KB) [Preprint] -
René Messina, Christian Holm, Kurt Kremer.
Polyelectrolyte Adsorption and Multilayering on Charged Colloidal particles.
Journal of Polymer Science, Part B: Polymer Physics 42:3557, 2004.
[PDF] (620 KB) [DOI] -
Juan J. Cerdà, Baofu Qiao, Christian Holm.
Understanding polyelectrolyte multilayers: an open challenge for simulations.
Soft Matter 5:4412–4425, 2009.
[PDF] (1.7 MB) [DOI] -
Juan J. Cerdà, Baofu Qiao, Christian Holm.
Modeling strategies for polyelectrolyte multilayers.
European Journal of Physics Special Topics 177(1):129–148, 2009.
[PDF] (971 KB) [DOI] -
Baofu Qiao, Juan J. Cerdà, Christian Holm.
Poly(styrenesulfonate)-Poly(diallyldimethylammonium) Mixtures: Toward the Understanding of Polyelectrolyte Complexes and Multilayers via Atomistic Simulations.
Macromolecules 43(18):7828–7838, 2010.
[PDF] (2.3 MB) [DOI] -
Baofu Qiao, Juan J. Cerdà, Christian Holm.
Atomistic Study of Surface Effects on Polyelectrolyte Adsorption: Case Study of a Poly(styrenesulfonate) Monolayer.
Macromolecules 44(6):1707–1718, 2011.
[PDF] (3.6 MB) [DOI]
References
[1] J. Schmitt, G. Decher and G. Hong. Thin Solid Films, 1992, 210/211, 831.URL
[2] G. Decher, Science,1997, 277, 1232. URL
[3] J. B. Schlenoff, Langmuir, 2009, 25, 14007.URL
[4] A. V. Dobrynin. Curr. Opin. Colloid Interface Sci, 2008, 13, 376.URL
[5] H. H. Hariri, and J. B. Schlenoff, Macromolecules, 2010, DOI: 10.1021/ma1012978. URL