Difference between revisions of "Nucleation in charged systems"
| Line 14: | Line 14: | ||
This will help in developing a closed theory of protein and colloidal crystallization and thereby making targeted crystallization possible. | This will help in developing a closed theory of protein and colloidal crystallization and thereby making targeted crystallization possible. | ||
| + | [[Category:Research]] | ||
Revision as of 15:23, 10 August 2011
The crystallization of charged macromolecules has a number of important applications in many fields, such as biology, pharmacology or materials design. For example, proteins are crystallized for purification or structure determination and colloidal crystals are promising candidates for photonic crystals.
However, the crystallization of proteins or colloids is still more an art rather than a technique due to the poor understanding of the underlying physical mechanisms. Typically, specific proteins are simply crystallized by a shotgun approach, that is, trying hundreds of combinations of precipitation agents at different conditions until a crystal is obtained.
To create defect-free crystals (or to prevent their growth), it is necessary to know the microscopic details of the onset of crystal growth, namely the nucleation. Fig. 1 shows schematically the formation of a nucleus at the early phase of crystal growth. The nucleation process is described by the classical nucleation theory (CNT) as a balance of surface tension between liquid and crystal and the crystal's lower chemical potential.
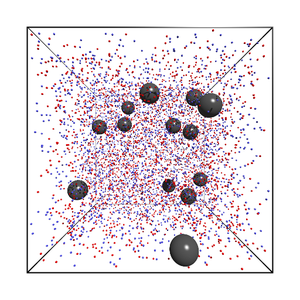
Aim of the project is to use computer simulations to gain a better understanding of how charges carried by the macromolecules and the presence of neutralizing salt and precipitation agents influence nucleation (Fig. 2).
To this aim, we study the input parameters for the CNT, namely surface tension and chemical potential and compare to direct simulations of nucleation. Since nucleation is a rare event, it is inaccessible to brute force computer simulations. Therefore, we apply a new sampling technique, Forward Flux Sampling (FFS), which has only recently become available.
This will help in developing a closed theory of protein and colloidal crystallization and thereby making targeted crystallization possible.